Introduction:
Buffer Solution, Picture this: You’re in a bustling laboratory, surrounded by vials, beakers, and the hum of scientific inquiry. Amidst this controlled chaos, a humble yet mighty solution quietly plays its role, maintaining stability amidst the chemical dance. Welcome to the world of buffer solutions – the unsung heroes of pH balance.
What Exactly is a Buffer Solution?
Let’s break it down in simpler terms. Imagine you’re at a concert, and there’s a crowd surging forward. The security guards form a barrier to prevent the chaos from overwhelming the stage. In a similar fashion, buffer solutions act as the guardians of pH balance. They’re a mix of two key players: a weak acid and its trusty companion, the conjugate base (or vice versa). Together, they form a dynamic duo, ready to tackle any pH disturbance that comes their way.

Ingredients of Buffer Solutions:
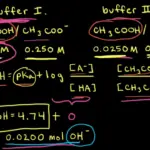
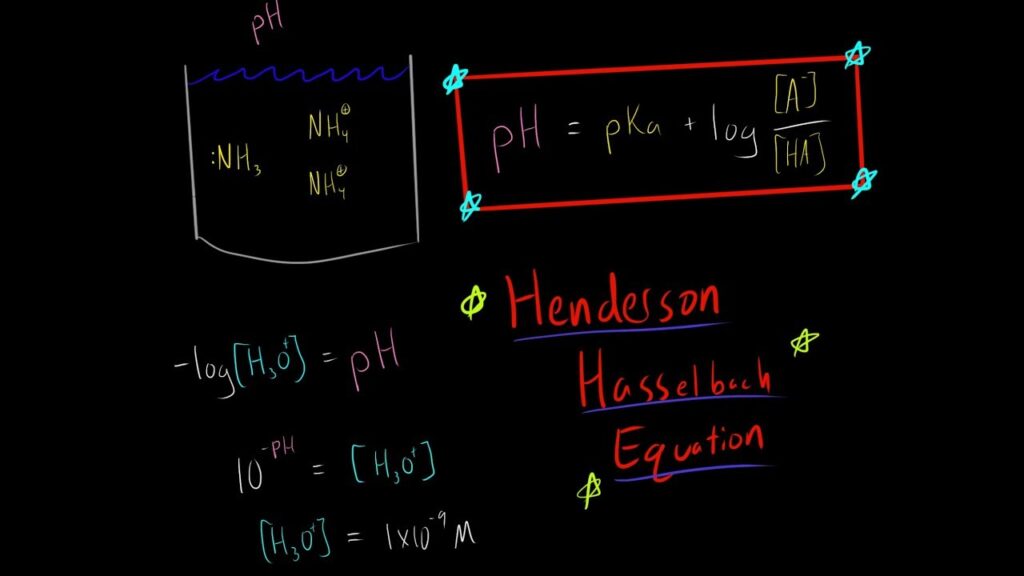
Think of a buffer solution like a well-rehearsed recipe – each ingredient plays a crucial role. You’ve got your weak acid, which is like the main actor, and its sidekick, the conjugate base. The Henderson-Hasselbalch equation serves as our culinary guide, helping us balance the pH just right.
How Buffers Work Their Magic:
Now, let’s see them in action. Imagine you’re in a boat, and suddenly, a wave hits. Instead of capsizing, the boat adjusts its balance, keeping you steady. That’s precisely what buffer solutions do when faced with an acidic or basic onslaught. They react strategically, neutralizing the threat and keeping pH levels in check.
Related Topic=Magnesium Sulfate Boiling Point: A Comprehensive Guide for Everyday Explorers
Why Buffer Solutions Matter:
- Biological Harmony: Our bodies are a delicate symphony of chemical reactions, all orchestrated within a narrow pH range. Buffer solutions ensure that this harmony isn’t disrupted, keeping our blood, saliva, and cellular fluids in tip-top condition.
- Pharmaceutical Guardians: Ever wondered why your medicine doesn’t taste too sour or too bitter? Thank the buffer solutions! They ensure that pharmaceutical formulations remain stable, potent, and palatable, making your healthcare experience smoother.
- Laboratory Allies: In the realm of research, precision is paramount. Buffer solutions lend a helping hand, providing a stable environment for experiments ranging from DNA analysis to protein purification. They’re the unsung heroes behind groundbreaking discoveries.
- Industrial Stabilizers: From brewing beer to treating wastewater, industrial processes rely on buffer solutions to maintain optimal pH conditions. They’re the silent guardians, ensuring that chemical reactions proceed smoothly without veering off course.
- Environmental Guardians: Water is life, and buffer solutions play a vital role in preserving its quality. By stabilizing pH levels in environmental samples, they enable accurate assessment of water quality, helping us protect fragile ecosystems.
Common Buffer Systems:
Let’s talk flavors! Just like a chef selects the perfect seasoning for a dish, scientists choose buffer systems tailored to their needs. Whether it’s the tangy acetic acid/acetate buffer or the mellow phosphate buffer, each has its own unique pH range and applications.
FAQs
1. What exactly is a buffer solution?
A buffer solution is a special mixture designed to maintain a stable pH level when an acid or base is added to it. It typically consists of a weak acid and its conjugate base, or a weak base and its conjugate acid.
2. How do buffer solutions work?
Buffer solutions work through a dynamic equilibrium between the weak acid and its conjugate base (or weak base and its conjugate acid). When an acid or base is added to the buffer, it reacts with the conjugate base or acid, respectively, minimizing changes in pH.
3. Why are buffer solutions important?
Buffer solution are crucial in various fields such as biology, chemistry, pharmaceuticals, and environmental science. They help maintain stable pH levels in biological systems, ensure the efficacy and stability of pharmaceutical formulations, facilitate precise laboratory experiments, and support industrial processes.
Conclusion:
Buffer solution may seem like humble mixtures, but their impact reverberates across scientific disciplines and industries. They’re the silent guardians of pH balance, ensuring stability amidst the chemical tumult. By understanding their composition, mechanisms, and applications, we gain a deeper appreciation for these unsung heroes. So, the next time you encounter a buffer solution, remember – it’s not just a liquid, but a silent sentinel, steadfast in its mission to maintain equilibrium amidst the chaos of chemistry.

Hello Everyone!! This is Sonia Inam. I am Chemist, have Done Masters in Chemical Sciences (along with ADS). I Basically Majored in all the Chemical Sciences. I Have worked as a Research Chemist on the “Structure and Bonding in Metal” Project. Hopefully, I Can be of some help to People on the Forum.