Introduction:
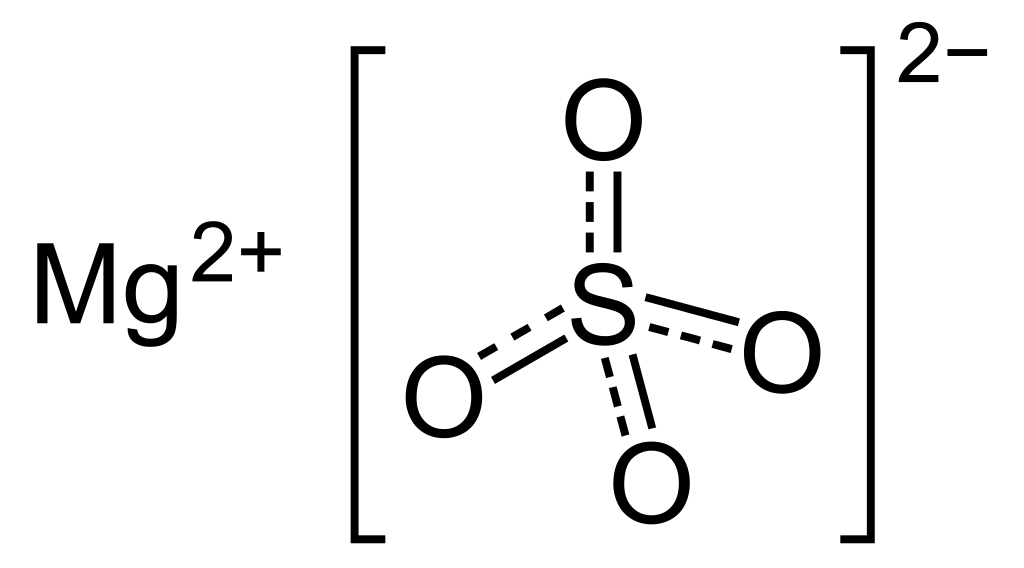
Magnesium Sulfate Boiling Point to the fascinating realm of magnesium sulfate, where science meets everyday life in unexpected ways. Magnesium sulfate, affectionately known as Epsom salt, is a compound that has woven itself into the fabric of our daily routines, from soothing baths to agricultural practices. But what about its boiling point? Let’s embark on a journey to unravel the mysteries of magnesium sulfate boiling point and uncover its significance in our lives.

Magnesium Sulfate Unveiled:
Picture this: you’re soaking in a warm bath, the stresses of the day melting away. That soothing sensation? You can thank magnesium sulfate for that. But beyond its role in relaxation, magnesium sulfate plays a crucial role in various industries, from healthcare to agriculture. Understanding its properties, including its boiling point, adds depth to our appreciation of this remarkable compound.
The Boiling Point Chronicles:
Now, let’s dive into the heart of the matter: the boiling point of magnesium sulfate. At its core, the boiling point signifies a transformation—a moment when a substance transitions from a liquid to a gas, dancing freely into the atmosphere. But for magnesium sulfate, this journey is influenced by several factors, each adding layers to its story.
Related Topic=Cardarine Side Effects
Factors Shaping the Boiling Point Odyssey:
- Concentration Clues: Imagine a solution brimming with magnesium sulfate molecules, each vying for attention. The more crowded the solution, the higher the boiling point. It’s a delicate dance of intermolecular forces, with concentration as the conductor guiding the symphony.
- Purity Ponderings: In the purity of Magnesium Sulfate Boiling Point lies its essence—a pristine canvas awaiting exploration. Impurities disrupt this harmony, casting shadows on its boiling point. Pure forms of magnesium sulfate offer a clearer path, a beacon of predictability amid uncertainty.
- Pressure Perspectives: Picture the atmosphere as a silent observer, silently shaping the destiny of magnesium sulfate. Under its watchful gaze, pressure dictates the ebb and flow of boiling points. Higher pressure raises the bar, while lower pressure beckons a gentler descent.
- Solvent Stories: Enter the world of solvents, where magnesium sulfate finds its allies in the dance of dissolution. Each solvent whispers secrets, altering the course of boiling points with its unique charm. It’s a tale of compatibility, where chemistry meets destiny.
Applications Unveiled:
Now that we’ve uncovered the nuances of magnesium sulfate boiling point, let’s explore its applications in our everyday lives:

- Soothing Salts for the Soul: In the quiet moments of self-care, magnesium sulfate bath salts offer solace—a sanctuary for weary souls seeking refuge from the chaos. Understanding its boiling point ensures the perfect temperature for a blissful soak.
- Nourishing Nature: Across vast fields, farmers sow the seeds of tomorrow, nourished by the wisdom of magnesium sulfate. Its boiling point guides the preparation of fertilizers, enriching the soil and nurturing the promise of bountiful harvests.
- Chemical Chronicles: In laboratories brimming with possibility, magnesium sulfate dances in the crucible of chemical reactions. Its boiling point orchestrates the symphony of transformations, a silent conductor shaping the destiny of molecules.
- Industrial Ingenuity: From textile mills to ceramic studios, magnesium sulfate lends its magic to industrial endeavors. Its boiling point sets the stage for innovation, fueling the imagination of artisans and engineers alike.
FAQs
1. What is the boiling point of magnesium sulfate?
The boiling point of magnesium sulfate varies depending on factors such as concentration, purity, pressure, and solvent. In general, it typically ranges from approximately 1450°C to 1520°C (2642°F to 2778°F).
2. How does the concentration of magnesium sulfate affect its boiling point?
Higher concentrations of magnesium sulfate in a solution tend to result in a higher boiling point. This is due to increased intermolecular forces between magnesium sulfate molecules, requiring more energy to transition to the gas phase.
3. Does the purity of magnesium sulfate influence its boiling point?
Yes, the purity of magnesium sulfate can impact its boiling point. Impurities in the compound can alter its properties, including its boiling point. Pure magnesium sulfate tends to have a more predictable boiling point compared to impure forms.
Conclusion:
In the tapestry of everyday life, Magnesium Sulfate Boiling Point emerges as a thread weaving through our experiences, connecting the dots between science and soul. By unraveling its mysteries and embracing its significance, we embark on a journey of discovery—a journey enriched by the wonders of magnesium sulfate and its enduring presence in our lives. So, let’s continue to explore, to marvel, and to cherish the remarkable world of magnesium sulfate, one boiling point at a time.

Hello Everyone!! This is Sonia Inam. I am Chemist, have Done Masters in Chemical Sciences (along with ADS). I Basically Majored in all the Chemical Sciences. I Have worked as a Research Chemist on the “Structure and Bonding in Metal” Project. Hopefully, I Can be of some help to People on the Forum.