Introduction:
Is melting ice a chemical change? Ice melting is like a dance of molecules, a mesmerizing transformation that occurs all around us. But beyond its beauty lies a question: Is melting ice merely a mundane physical event, or does it hold secrets of chemical change? Join us as we embark on a journey to unravel the mysteries of ice melting, blending science with the marvels of everyday life.
The Heart of Chemistry: Understanding Chemical Change
Before we delve into the specifics of ice melting, let’s take a moment to grasp the essence of chemical change. It’s like the alchemy of nature, where substances undergo magical transformations at the molecular level, giving birth to new identities with unique properties.
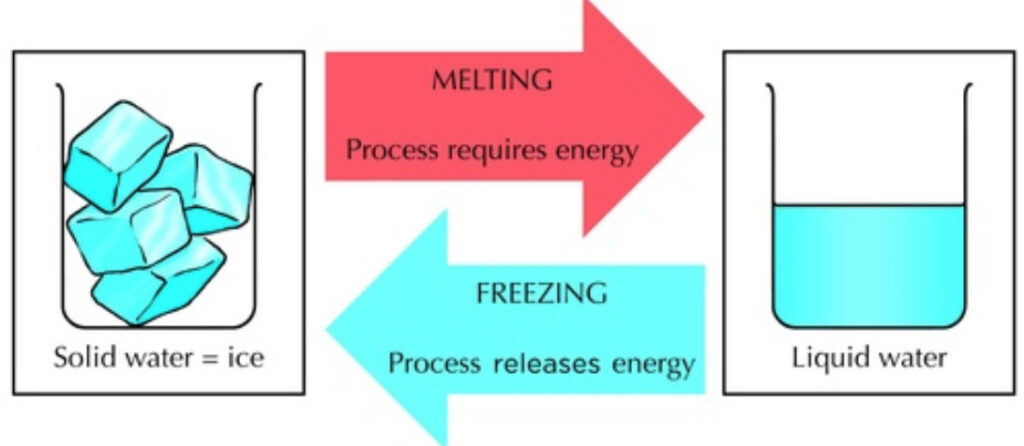
The Enchantment of Ice Melting: A Symphony of Physical Transitions
Imagine a world where ice isn’t just frozen water but a gateway to transformation. When ice melts, it’s more than just a physical shift—it’s a symphony of molecules breaking free from their icy bonds and embracing the fluidity of life.
a. Molecular Ballet: Picture water molecules locked in a crystalline embrace, held together by delicate hydrogen bonds. As heat whispers its warm breath, these molecules begin their graceful dance, breaking ranks and slipping into a fluid embrace.
b. A Dance of Reversibility: Unlike the irreversible dramas of chemical change, ice melting is a tale of reversibility. When the temperature drops, the fluidity retreats, and the molecules find solace once again in the crystalline arms of ice.
c. The Elegance of Conservation: In the grand theater of chemistry, mass is a sacred principle. In the realm of ice melting, this principle holds—the total mass of water remains a constant, a testament to the conservation of matter.
Related Topic=Chemical Waste Disposal
Unveiling the Sorcery: Factors Shaping Ice Melting
Ice Melting is a delicate waltz influenced by a myriad of factors, each adding its brushstroke to the canvas of transformation:
a. Temperature: Imagine temperature as the conductor of this symphony, directing the pace of molecular motion. With a gentle rise in temperature, the ice surrenders to the warmth, melting into a liquid embrace.
b. Pressure’s Embrace: Sometimes, pressure joins the dance, whispering secrets to the ice. Under its tender touch, the melting point bends, revealing new dimensions of fluidity and grace.
c. Impurities: And then there are the silent companions—the impurities that color the ice with their presence. Salt, and minerals, all play their part, altering the melody of melting through a dance of freezing point depression.
Echoes of Melting: Where Science Meets Life
Ice melting isn’t just a tale told in laboratories; it’s a narrative woven into the fabric of our lives:

a. Tales of Preservation: In kitchens around the world, ice melting guards the treasures of food, preserving flavors and memories in a cold embrace.
b. Whispers of Climate Change: But beyond our homes, the melting of polar ice caps and glaciers whispers ominous truths about our changing climate, urging us to heed the warnings of nature’s symphony.
c. Dreams of Innovation: In the halls of innovation, engineers and scientists harness the power of ice melting, designing structures, vehicles, and materials that brave the icy winds of winter with grace and resilience.
FAQs
Is melting ice a chemical change or a physical change?
Melting ice is primarily a physical change rather than a chemical change. It involves a transition from the solid phase (ice) to the liquid phase (water) without any alteration in the chemical composition of the molecules involved.
What causes ice to melt?
Ice melts when it absorbs heat energy from its surroundings. This added energy increases the kinetic energy of the water molecules in the ice, causing them to overcome the intermolecular forces that hold them in a rigid structure, thus transitioning into the liquid phase.
Can ice melt at room temperature?
Yes, ice can melt at room temperature if the surrounding environment is warm enough. The melting point of ice is 0 degrees Celsius (32 degrees Fahrenheit), so any temperature above this threshold can cause ice to melt.
Conclusion:
In the end, ice melting is more than just a scientific phenomenon—it’s a poem written in the language of molecules, a dance of transformation that captivates our imagination and enriches our understanding of the world around us. So, the next time you witness the magic of ice melting, let it be a reminder of the beauty and wonder that lie hidden in the simplest of phenomena.

Hello Everyone!! This is Sonia Inam. I am Chemist, have Done Masters in Chemical Sciences (along with ADS). I Basically Majored in all the Chemical Sciences. I Have worked as a Research Chemist on the “Structure and Bonding in Metal” Project. Hopefully, I Can be of some help to People on the Forum.





1 thought on “Is melting ice a chemical change”